Update on COVID-19 Testing and the Medical Supply Chain
How the medical supply chain is impacting COVID 19 testingWhy Test? What is the COVID-19 Testing Strategy?
Early in the pandemic, the scientific community advised that COVID-19 testing would be instrumental in getting the spread of the coronavirus under control. The strategy of imposing lockdowns was designed to stall for time so that the virus would have only minimal spread. It was anticipated that coronavirus outbreaks could be able to be maintained at sufficiently low levels by leveraging significant testing, tracing of those infected and mandatory quarantines. This would be able to stop the forward transmission of the virus.
The point of testing is to determine the onset of an infection so action can be taken to stop the forward spread of COVID-19. For this to be an effective strategy, tests would have to be readily available across the United States in widespread locations, from rural to urban and the results would need to be provided quickly.
Problems with COVID-19 Tests
With respect to the effectiveness of the testing strategy, one issue persists: there still is no “gold standard”, a test by which the accuracy of all other COVID-19 detection tests can be measured. The challenge in this lies in determining the sensitivity and specificity of the tests. Although the FDA has provided laboratories with comparative data which determines the limit of detection for several tests, this information has had limited usefulness.
Many different tests were developed from point-of-care rapid tests, small, self-contained tests that do not require instrumentation to PCR analyzers and many more innovations. Testing types differed in the reliable accuracy rates, processing times and other factors.
The lack of a federally coordinated COVID-19 response plan was a significant reason why the testing program failed. Overall, the U.S. lacked the ability to conduct widespread testing of the population and receive rapid, accurate results. This greatly hampered the ability to identify where the coronavirus was spreading and inhibited the ability to strategically execute efforts to reduce the spread. For example, if widespread, fast, highly accurate testing were universally available across both rural and highly populated geographic areas of the United States, scientists could identify outbreaks, arrange for contact testing to find the sources of the infection, and quarantines, lockdowns and other restrictions could be imposed only on the affected areas rather than across a wide, generalized population.
COVID-19 testing in the U.S. remains a patchwork effort. Turnaround times for COVID-19 testing results usually span several days. Without knowing their infection status, patients often encounter situations and people which pose new risks, and often unknowingly, pass on the coronavirus. As many people test possible but are asymptomatic, this is very understandable. Not knowing that a person is positive for the coronavirus can be deadly, allowing the infection to take hold and spread to other people.
Another issue involved with COVID-19 testing is the inaccuracy of rapid response tests. Sole reliance on rapid COVID-19 tests without taking other measures advised by the CDC including properly wearing a facial mask and washing hands, social distancing and refraining from touching the face, eyes, etc. is unlikely to provide any protection or guarantee of results against the coronavirus.
While at home tests have been developed, they require shipment to a laboratory, often far away from the patient location. Processing the tests at these labs quickly is often stalled by shipping and delivery time as well as by any backlog at the respective lab. At home tests may be problematic for another reason. While they are simple to administer, some have specifications or special instructions such as that the patient must refrain from eating or drinking for a certain period of time prior to testing, etc. Failure to properly adhere to directions impacts results.
Medical Testing: Medical Equipment and Medical Supplies
Many community hospitals across the U.S. use the Cepheid machine for processing laboratory tests in general. Hospital do tend to adapt existing machines for running COVID-19 tests. Lab instruments including the Cepheid are on back order as are the supplies required to be used with the machine to properly conduct testing. Medical machine manufacturers did not expect at the beginning of the year to have to ramp up production and this process takes time and money.
Over the summer, as more sites were opened to allow COVID tests to be processed, specialized test cartridge containing a reagent were lacking crucial components of the tests, causing shortages of complete COVID-19 test kits across the country. When facilities ran out of in-house testing kits, outside labs had to be used. This meant that results for coronavirus tests took days to process, instead of mere minutes to get results. This not only impacted COVID patients but also patients waiting for elective surgeries, such as hip or knee replacement surgeries.
Outside laboratories have often been more successful in obtaining test materials as compared to hospital labs because the United States runs on a privatized health system. Test manufacturers are often more inclined to sell testing materials and kits in huge quantities.
Medical Supply Chain Challenges of COVID-19 Testing Resources
The American Association for Clinical Chemistry ran a national survey to learn about the challenges faced with laboratory testing. Approximately half of the laboratories reported struggles in obtaining reagents or testing swabs. Some of the problems identified with these efforts include supply chain problems, distribution challenges and lack of government oversight.
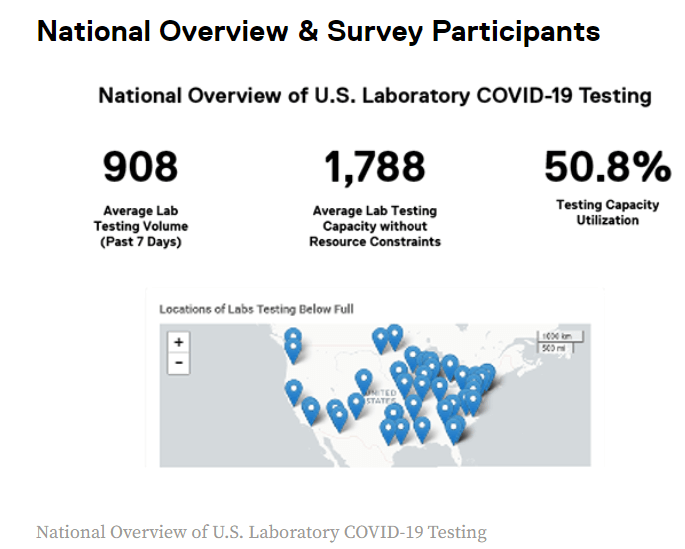
The American Society for Microbiology (ASM) began collaborating with the Association of Supply Chain Management (ASCM) on gathering testing medical supply chain status for COVID-19 and other microbiological tests. As of September 11th, the ASM initiated the independent gathering of shortage data directly from clinical laboratories. Initially 134 CLIA laboratories participated in the recent period (November 10-17, 2020) and reported an average testing capacity of 50.8% for COVID-19. This demonstrates that in this group of respondents, there is still a noticeable supply shortage.
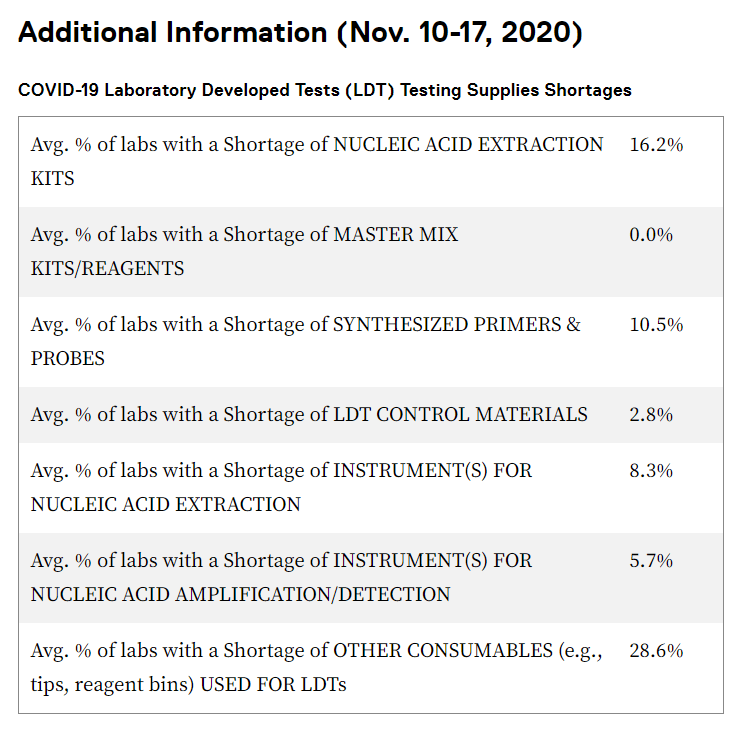
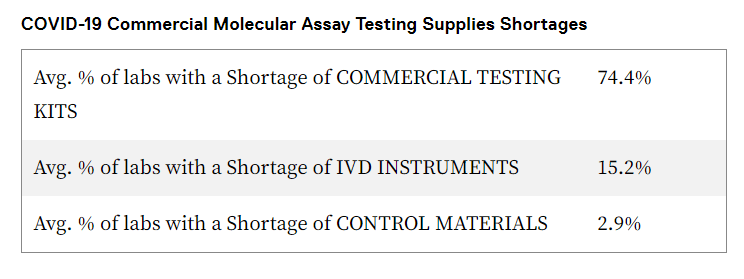
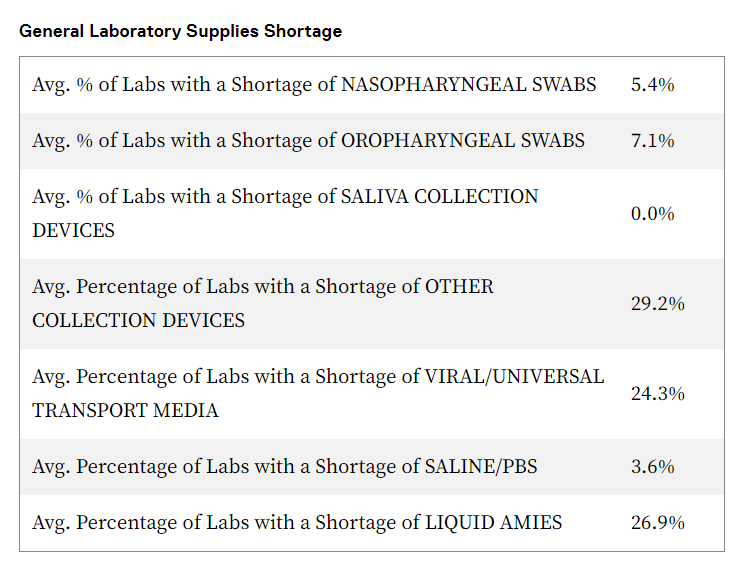
Here are some of the supply chain-related challenges:
- Lack of reliable access to COVID-19 testing kits, machines and/or reagents needed to process the tests and for routine patient care on a daily basis
- Shortages of laboratory medical supplies needed for processing of routine tests that are unrelated to COVID-19:
- Culture and transport media
- Swabs
- Pipettes
- Pipette tips
- Collection tubes
- Needles and syringes, such as those used to give vaccinations
- Blood agar, an enriched culture medium used to grow hundreds of differing kinds of bacteria to test for infections
- Mueller Hinton (MH) media, a growth medium used to measure the susceptibility of antibiotics to different types of bacteria, impacting the ability to prescribe the appropriate antibiotics for patient infections
- Other growth mediums including but not limited to chocolate agar, fungal culture media, viral transport media.
Reference labs, usually private, commercial facilities that perform high volume routine and specialized testing procedures, are often overloaded by the high volume of samples they are receiving. Because of these conditions, some labs either refuse to accept new samples or the processing time is extended more than 7 days.
With the continued escalation in volume of COVID-10 testing, clinical testing suppliers and manufacturers have been forced to redistribute resources and staff to enable them to prioritize the manufacturing, collection and transport of goods needed for COVID-19 testing. During the early days of the pandemic, some research labs were obliged to minimize their research or shut down. When this occurred, the demand for plated media declined significantly. As this is inventory that expires, it had to be destroyed. In addition, the amount of non-COVID supplies manufactured recently has declined.
Laboratories are having to contend with unforeseen, unpredictable and sometimes unprecedented supply chain issues which keep changing. As there is no consistent, centralized federally coordinated response, states, health care systems and other parties are forced to bid against each other for medical supplies that are in high demand.
Studies have found that supply chain challenges have been especially problematic for smaller, regional testing sites. Often companies selling medical supplies had high minimum order quantities that were totally out of reach for a small county or rural region.
Conclusion:
Laboratories across the United States have been reporting shortages in essential medical supplies needed not only for COVID-19 tests but also for typical ailments and diseases experienced by patients. The result of longer testing turnaround times, less frequent testing may mean that physicians are less able to accurately diagnose and treat disease-causing pathogens. It may also mean that more broad-spectrum antibiotics are used because specific viruses and bacteria cannot be necessarily identified. Overuse of antibiotics may also become a more significant problem.
In addition, patients may be misdiagnosed and prescribed medical treatments that are inappropriate, harmful or ineffective, leading to pain, suffering and even death. Lack of appropriate laboratory resources leads to more manual testing which is subject to human error.
Testing and reporting are the backbone of the COVID-19 strategy. At any given time, health care professionals and the public need to know how many intensive care beds are available, patients have tested positive for the novel coronavirus (COVID19) and how many people are hospitalized, died, etc. Without quick, accurate testing, scientists and health care professionals are unable to be adequately prepare, follow through with track and trace and contain the novel coronavirus. Every day that hospital supply chains are unable to procure the necessary medical equipment and medical supplies, the spread of COVID19 increases.
Global supply chain challenges during COVID19 have undoubtedly been problematic and need to be resolved as comprehensively and quickly as possible before more lives are lost.
Next in this series:
Personal Protective Equipment and the Medical Supply Chain
Still in the throes of the COVID19 pandemic, the need for protective equipment for medical professionals is acute and remains a matter of grave concern. Is the PPE supply adequate to ensure a healthy community of health care professionals? Are hospital supply chains holding steady? Resorting to nontraditional suppliers of personal protective equipment?
What about personal protective gear for other care settings such as care facilities for the elderly? Are health care systems that leverage health care professionals for in home care finding it challenging to procure an adequate supply of medical protective equipment for those health care professionals?
Are N95 masks still the preferred PPE for healthcare professionals? How are global supply chains managing the tremendous need for PPE during the COVID19 outbreak?
Stay tuned.
What Makes Datex Different?
1. Revolutionary low code/no code flexible workflow-driven warehouse management software
2. Most configurable, user-friendly WMS on the market today
3. End-to-end solution provider: software, hardware, EDI, and managed services
4. White Glove Concierge Service
5. Executive-level attention and oversight